Jun 28, 2018 | Blog, Medical Marijuana, News
Right now smoking medical cannabis prevents people from benefiting from federal housing.
The “Marijuana in Federally Assisted Housing Parity Act of 2018” introduced by Rep. Eleanor Holmes Norton (D-D.C.) will protect people who use medical marijuana.
It’s an ambitious bill prompted by a D.C. resident’s need for cannabis medication who lives in federally assisted housing.
Currently no matter the state law using cannabis products blocks someone from benefiting from federal housing which in many cases means the difference between the streets and a home.
The D.C. resident’s name is Sondra Battle and Rep. Homes Norton is calling the Bill, “Sondra Battle Cannabis Fair Use Act.” Clearly, the Representative is greatly moved by Battle’s story and sees the healing benefits of cannabis as big enough to supersede Federal law in states that already allow for medical or recreational marijuana.
Rep. Holmes Norton declared, “Residents like Sondra should not fear eviction from federally assisted housing simply for using cannabis to treat their medical conditions.”

Jun 27, 2018 | Blog, Medical Marijuana, News
The Senate passed the bill by a vote of 86 to 5 for U.S. military veterans who would be permitted to be given recommendations for medical marijuana from government doctors under new legislation.
This provision is part of a legislation to fund parts of the federal government including the Department of Veterans Affairs (VA) .
This would also protect veterans from losing their government benefits as a result of cannabis use that is legal under state law.
Read More

Jun 25, 2018 | Michigan Medical Marhuana Regulation, News
Statement by FDA Commissioner Scott Gottlieb, M.D., on the importance of conducting proper research to prove safe and effective medical uses for the active chemicals in marijuana and its components.
Over the past decade, we’ve seen a growing interest in the development of therapies derived from marijuana and its components. Proponents of “medical marijuana” advertised its uses for a wide number of medical conditions, such as cancer, multiple sclerosis, post-traumatic stress disorder and anxiety – just to name a few of the touted conditions. The FDA has been supportive of research in this area for many years. But marijuana is a Schedule I compound with known risks. Research to demonstrate that marijuana or its components could be safe and effective in the treatment of medical disorders should be held to the same standard as other drug compounds. And certainly it should not be held to a lower standard, as some proponents would suggest. The FDA has an active program to assist drug developers who want to investigate marijuana or its components through properly controlled clinical trials, to demonstrate the potential for safe and effective uses.
Today, the FDA approved a purified form of the drug cannabidiol (CBD). This is one of more than 80 active chemicals in marijuana. The new product was approved to treat seizures associated with two rare, severe forms of epilepsy in patients two years of age and older.
This product approval demonstrates that advancing sound scientific research to investigate ingredients derived from marijuana can lead to important therapies. This new treatment provides new options for patients.
This is an important medical advance. But it’s also important to note that this is not an approval of marijuana or all of its components. This is the approval of one specific CBD medication for a specific use. And it was based on well-controlled clinical trials evaluating the use of this compound in the treatment of a specific condition. Moreover, this is a purified form of CBD. It’s being delivered to patients in a reliable dosage form and through a reproducible route of delivery to ensure that patients derive the anticipated benefits. This is how sound medical science is advanced.
So today, in addition to celebrating this scientific achievement and the medical advance that it represents for these patients and their families, we should also reflect on the path that made this possible. It’s a path that’s available to other product developers who want to bring forth marijuana-derived products through appropriate drug development programs.
That pathway includes a robust clinical development program, along with careful review through the FDA’s drug approval process. This is the most appropriate way to bring these treatments to patients. This process also includes a review of the purity of a new drug and manufacturing controls. Before a high-quality drug can be developed, evaluated, and eventually approved by the FDA; it’s critical that the necessary work is done to identify drugs of potential medical benefit and conduct rigorous scientific research through adequate and well-controlled clinical trials. This is true for all drugs, including ones derived from plant materials, like marijuana. And the FDA remains committed to collaborating with federal and state agencies, researchers and product developers on advancing this type of important and conscientious work.
This research process – from early development through preclinical and clinical research – gives us a comprehensive understanding of a new drug. That includes an understanding of whether the new product is safe and effective for treating a particular medical condition, what the proper dosage is and for what populations it is safe and effective, how the new compound could interact with other drugs, or whether the new drug has side effects or other safety concerns.
This work also helps product developers identify the appropriate dosage needed to achieve the desired therapeutic effect while minimizing toxicity and risk. Taken in totality, the scientific evidence generated by these studies forms the basis of the FDA’s evaluation of benefit versus risk. And it’s because of this careful, scientific and evidence-based evaluation by the FDA that health care providers can rely on having a quality product that delivers a consistent, uniform dose of an effective medication that is able to deliver a predictable treatment to patients. This is especially important when considering treatment for serious medical conditions that will be utilized in the clinical care of patients who may have any number of health vulnerabilities. The purified form of the drug CBD approved today by the FDA has been shown to meet these rigorous standards.
Research on the therapeutic effects of marijuana and its components involves a number of federal agencies in addition to the FDA, including the National Institute on Drug Abuse, part of the National Institutes of Health, and the Drug Enforcement Administration.
The FDA has taken several specific steps to support this research.
We meet regularly with researchers as they plan and carry out their trials. We have also formed a Botanicals Team that provides scientific expertise on botanical issues for researchers developing drugs derived from plants, such as marijuana. That team published guidance for industry on clinical studies involving botanical drugs, as well as quality controls for lot-to-lot consistency. In recent years, the agency also has recommended to the DEA the approval of several hundred Schedule I research protocol licenses for research on marijuana or its constituent compounds.
Additionally, the FDA also works with companies to provide patients access to experimental therapies while clinical trials are ongoing through expanded access provisions. These approaches help protect patients while also allowing for the collection of data necessary to support the FDA approval of safe and effective therapies for use in the broader population. Through this process, hundreds of children were able to get access to investigational CBD products while this product was being studied.
Drugs derived from marijuana also are eligible for several programs that are intended to facilitate and expedite development and review of new drugs that address unmet medical needs in the treatment of serious or life-threatening conditions. Much of the work we’ve done to encourage research in this area has led to the approval action we took today.
The FDA will continue to support rigorous scientific research on potential medical treatments using marijuana and its components that seek to be developed through the appropriate scientific channels. However, we remain concerned about the proliferation and illegal marketing of unapproved CBD-containing products with unproven medical claims.
The promotion and use of these unapproved products may keep some patients from accessing appropriate, recognized therapies to treat serious and even fatal diseases. The FDA has taken recent actions against companies distributing unapproved CBD products. These products have been marketed in a variety of formulations, such as oil drops, capsules, syrups, teas, and topical lotions and creams. These companies have claimed that various CBD products could be used to treat or cure serious diseases such as cancer with no scientific evidence to support such claims. We’ll continue to take action when we see the illegal marketing of CBD-containing products with unproven medical claims. We’re especially concerned when these products are marketed for serious or life threatening diseases, where the illegal promotion of an unproven compound could discourage a patient from seeking other therapies that have proven benefits.
Today’s approval demonstrates our commitment to the scientific process and working with product developers to bring marijuana-based products to market. We remain committed to our gold standard for product development and review. Such a process ensures that any new therapies from marijuana and its constituents are safe, effective and manufactured to a high and consistent quality. And most importantly, that these products have been proven safe and effective for patients.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.

Jun 24, 2018 | Blog, Legalization, News
Recreational marijuana use will soon be legal in Canada after the Senate passed a “historic” bill on Tuesday 6/19/18 with a vote of 52-29.
Canada is the second country in the world to implement legislation to permit a nationwide marijuana market.
Uruguay was the first country to legalize marijuana’s production, sale and consumption in December 2013. Read the law and regulation-if you can here
In the neighboring US, nine states and the District of Columbia now allow for recreational marijuana use, and 30 allow for medical use.
The act to legalize the recreational use of marijuana was introduced on April 13, 2017, and was later passed at the House of Commons in November. The Senate passage of the bill was the final hurdle in the process.
Bill C-45, AKA…The Cannabis Act, stems from a campaign pledge of Prime Minister Justin Trudeau to keep marijuana away from underage users and reduce related crime.
Although the Canadian government had initially stated its intent to implement by July 2018, provinces and territories and who would be responsible for drafting their own rules for marijuana sales. They have advised that they would need eight to 12 weeks after the Senate approval to transition to the new framework.
The government is expected to choose a date in early or mid September.

Jun 18, 2018 | Blog, Hemp, News
Senators Ron Wyden, D-Oregon, Mitch McConnell, R-Ky., Jeff Merkley, D-Oregon, and Rand Paul, R-Ky., cheered the passage of their bipartisan Senate resolution designating June 4-10 “Hemp History Week.”

Jun 18, 2018 | Blog, News
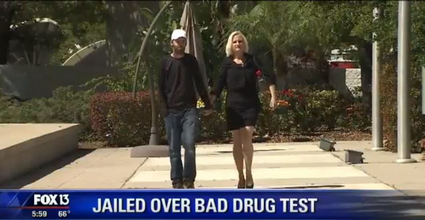
A mother with no criminal record of 4 separated from her family and children after being jailed for vitamins being mistaken for drugs in a widely used drug test kit by police.
These Duquenois Levine reagent tests for marijuana will test positive for most any substances, candies, even the air will make them change color and false positive alert for a controlled substance.
Never consent to a search by a police officer. Tell the officer you do not consent to a search and ask if you are free to go. Don’t answer any questions , tell the officer you want your lawyer present during any questioning. These important phrases will keep you out of trouble and protect your constitutional rights!