During this February 20, 2013 hearing, Assistant Oakland County Prosecutor Beth Hand notified the court that her office is contemplating filing criminal charges against a medical doctor for his involvement in certifying two medical marijuana patients, Robert Redden...

Trulieve seeks $143M federal refund for 280E taxes
Would enforcing payment and accepting money from a federally illegal business cause you to be caught up in RICO, CCE and conspiracy charges that would put you away for decades? For you yes – For the government a big NO.
Multistate marijuana company Trulieve Cannabis Corp. is currently seeking a federal tax refund amounting to $143 million. The company firmly maintains that it does not owe the taxes it had diligently paid over a span of three years.
“This determination is supported by legal interpretations that challenge the company’s tax liability under Section 280E of the Internal Revenue Code,” Florida-based Trulieve announced through a recent news release.
Section 280E poses a significant obstacle for state-legal marijuana companies, as it prohibits them from deducting their standard business expenses. Consequently, these companies are burdened with substantially increased tax bills.
26 U.S. Code § 280E – Expenditures in connection with the illegal sale of drugs
No deduction or credit shall be allowed for any amount paid or incurred during the taxable year in carrying on any trade or business if such trade or business (or the activities which comprise such trade or business) consists of trafficking in controlled substances (within the meaning of schedule I and II of the Controlled Substances Act) which is prohibited by Federal law or the law of any State in which such trade or business is conducted.
More Posts

People v Redden & Clark – MI Medical Marijuana hearing – February 20 2013

Medical marijuana lawyers want state crime lab moved out of Michigan State Police
"The attorneys claim the policy change is leading to unfair felony charges for patients who would otherwise face misdemeanors." Posted on MichiganRadio.org A group of criminal defense attorneys says the Michigan State Police (MSP) should no longer...

Defense attorneys seek fed inquiry of MSP crime labs
Southfield — Three defense attorneys are asking the federal government to investigate the Michigan State Police crime laboratories, alleging misconduct in their testing for pending drug cases. Southfield defense attorneys Neil Rockind and Michael Komorn, along...

MI Cops Change Policy So They Can Falsely Imprison Legal Pot Smokers
In 2008, an overwhelming majority of Michigan voters approved legislation to legalize marijuana for medical use in the state. With nearly 50,000 Michigan residents arrested and incarcerated each year for controlled substance violations, the state’s prison industrial...

Attorney Alleges Authorities `Bend The Science’ To Elevate Marijuana Cases
MIRS-Michigan Independent Source Of News and Information Friday Nov 6, 2015 Maxwell LORINCZ, of Spring Lake, says a fingerprint of oil on an empty plastic container led to his arrest on a drug charge on Sept. 24, 2014. Now, a year later, the case that might have...
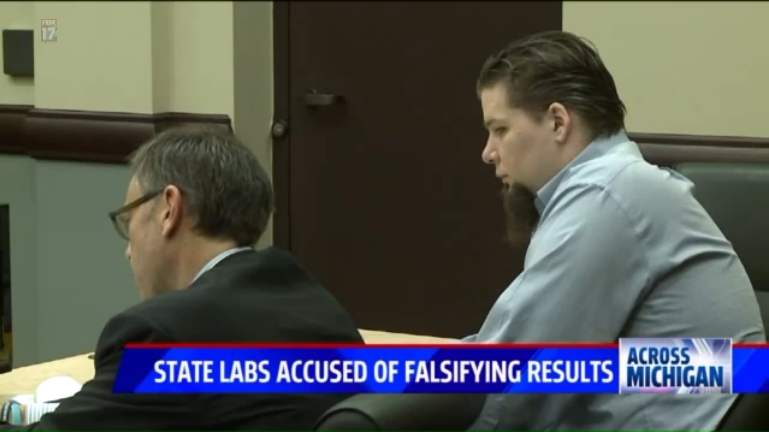
Drug felonies without credible proof? — Allegations of politicking in state police crime labs
GRAND RAPIDS, Mich. – First on FOX 17, we broke serious allegations that state police crime labs are being told to falsely report marijuana test results. This is resulting in misleading lab reports that an attorney claims creates felonies without real proof. ...

Attorney: Crime labs ‘falsified’ marijuana reports
A Southfield lawyer alleges the Michigan State Police crime labs have “falsified lab reports on marijuana statewide” and he’s asking a judge to dismisses charges lodged against a client. Michael Komorn, who also represents defendants in Livingston County, said...

Hearing in alleged false crime lab marijuana reporting dropped this week
OTTAWA COUNTY, Mich. – The evidentiary hearing originally set for Nov. 5 has been dropped in the case involving a medical marijuana patient charged with a disputed felony for synthetic THC, the psychoactive ingredient in marijuana. The Ottawa County Assistant...

“A non-stop political game:” Former MSP Forensic Science director on false marijuana reporting …
DEWITT, Mich. – A former director of Michigan State Police Forensic Science addressed the serious allegations FOX 17 uncovered, which accuse the Attorney General’s office and state prosecutors of influencing state police crime labs to falsely report marijuana;...

Michigan’s medical marijuana law circumvented by crime labs’ THC reports, attorney charges
Posted on MLive 10/30/15 OTTAWA COUNTY, MI – An attorney claims prosecutors pressured state police crime labs to change the way THC, the active ingredient in marijuana, is reported in an effort to circumvent Michigan's medical marijuana law. Michael...

























