The revised federal workplace drug testing guidelines, issued by the Substance Abuse and Mental Health Services Administration (SAMHSA), Department of Health and Human Services (HHS), are intended to provide clarity. These guidelines emphasize that individuals who use medical marijuana under a doctor’s recommendation in a legal state cannot use it as a valid justification for a positive THC test.
In notices published in the Federal Register, SAMHSA announced that it had amended guidance of saliva and urine testing to include the cannabis policy clarification, despite receiving comments opposing the proposal after they were first announced last year.
Mandatory Guidelines for Federal Workplace Drug Testing Programs
A Rule by the Health and Human Services Department on 10/12/2023
AGENCY:
Substance Abuse and Mental Health Services Administration (SAMHSA), Department of Health and Human Services (HHS).
ACTION:
Issuance of mandatory guidelines.
SUMMARY:
The Department of Health and Human Services (“HHS” or “Department”) has revised the Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG) which published in the Federal Register of October 25, 2019.
DATES:
The mandatory guidelines are effective October 10, 2023.
FOR FURTHER INFORMATION CONTACT:
Eugene D. Hayes, Ph.D., MBA, SAMHSA, CSAP, DWP; 5600 Fishers Lane, Room 16N02, Rockville, MD 20857, by telephone (240) 276–1459 or by email at Eugene.Hayes@samhsa.hhs.gov.
SUPPLEMENTARY INFORMATION:
Executive Summary
These revised Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG) establish a process whereby the Department annually publishes the authorized drug testing panel ( i.e., drugs, analytes, or cutoffs) to be used for Federal workplace drug testing programs; revise the definition of a substituted specimen to include specimens with a biomarker concentration inconsistent with that established for a human specimen, establish a process whereby the Department publishes an authorized biomarker testing panel ( i.e., biomarker analytes and cutoffs) for Federal workplace drug testing programs; update and clarify the oral fluid collection procedures; revise the Medical Review Officer (MRO) verification process for positive codeine and morphine specimens; and require MROs to submit semiannual reports to the Secretary or designated HHS representative on Federal agency specimens that were reported as positive for a drug or drug metabolite by a laboratory and verified as negative by the MRO. In addition, some wording changes have been made for clarity and for consistency with the Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG) or to apply to any authorized specimen type.
The Department is publishing a separate Federal Register Notification (FRN) elsewhere in this issue of the Federal Register with the revised UrMG, which include the same or similar revisions as the OFMG, where appropriate.
Background
Pursuant to its authority under section 503 of Public Law 100–71, 5 U.S.C. 7301, and Executive Order 12564, HHS establishes the scientific and technical guidelines for Federal workplace drug testing programs and establishes standards for certification of laboratories engaged in drug testing for Federal agencies.
Using data obtained from the Federal Workplace Drug Testing Programs and HHS-certified laboratories, the Department estimates that 275,000 urine specimens are tested annually by Federal agencies. No Federal agencies are testing hair or oral fluid specimens at this time.
HHS originally published the Mandatory Guidelines for Federal Workplace Drug Testing Programs (hereinafter referred to as Guidelines or Mandatory Guidelines) in the Federal Register (FR) on April 11, 1988 (53 FR 11979). The Substance Abuse and Mental Health Services Administration (SAMHSA) subsequently revised the Guidelines on June 9, 1994 (59 FR 29908), September 30, 1997 (62 FR 51118), November 13, 1998 (63 FR 63483), April 13, 2004 (69 FR 19644), and November 25, 2008 (73 FR 71858). SAMHSA published the current Mandatory Guidelines for Federal Workplace Drug Testing Programs using Urine (UrMG) on January 23, 2017 (82 FR 7920) and published the current Mandatory Guidelines for Federal Workplace Drug Testing Programs using Oral Fluid (OFMG) on October 25, 2019 (84 FR 57554). SAMHSA published proposed Mandatory Guidelines for Federal Workplace Drug Testing Programs using Hair (HMG) on September 10, 2020 (85 FR 56108) and proposed revisions to the UrMG (87 FR 20560) and OFMG (87 FR 20522) on April 7, 2022.
There was a 60-day public comment period following publication of the proposed OFMG, during which 53 commenters submitted 204 comments on the OFMG. These commenters were comprised of individuals, organizations, and private sector companies. The comments are available for public view at https://www.regulations.gov/. All comments were reviewed and taken into consideration in the preparation of the Guidelines. The issues and concerns raised in the public comments for the OFMG are set forth below. Similar comments are considered together in the discussion.
Summary of Public Comments and HHS’s Response
The following comments were directed to the information and questions in the preamble.
Some submitted comments were specific to transportation industry drug testing which is regulated by the Department of Transportation (DOT). The Department has noted these comments below, but responded only to comments that are relevant to these Guidelines. DOT issued a notice of proposed rulemaking (NPRM) on February 28, 2022 (87 FR 11156). Subsequently, DOT extended the comment period to April 29, 2022 (87 FR 16160), and published the final rule on May 2, 2023 (88 FR 27596).
Authorized Drug Testing Panel
The Department requested comments on its proposal to publish the drug testing panel separately from the OFMG in a Federal Register\[[[[p Notification (FRN) each year. Fifteen commenters submitted a total of 40 comments on this topic for the OFMG.
Nine commenters disagreed with publishing a revised drug testing panel without a public comment period, expressing concerns that stakeholders including individuals subject to federally regulated drug testing would not be given the opportunity to provide comment and that the Department would miss valuable input including information on costs and burden. Some of these commenters suggested alternate ways to permit public comment while enabling a quicker response to testing panel changes ( e.g., setting a shorter comment period, publishing the Guidelines as an interim final rule or issuing an advance notice of proposed rulemaking). The Department has reviewed these comments and suggestions and determined that no changes to the proposed Guidelines are needed. The Department has developed procedures which will allow review and comment before testing panel changes are published, as described below.
Consistent with current procedures, prior to making a change to the drug or biomarker testing panel, the Department will conduct a thorough review of the scientific and medical literature, and will solicit review and input from subject matter experts such as Responsible Persons (RPs) of HHS-certified laboratories, Medical Review Officers (MROs), research scientists, manufacturers of collection devices and/or immunoassay kits, as well as Federal partners such as DOT, the Food and Drug Administration (FDA), and the Drug Enforcement Administration (DEA). Further, the Department plans to provide notice and opportunity for public comment regarding any proposed changes to the drug and biomarker testing panels as part of Drug Testing Advisory Board (DTAB) meetings and procedures.
Information regarding any proposed changes to the drug and biomarker testing panels and a request for public comment will be included in an advance notice of the DTAB meeting published in the Federal Register , along with the timeframe and method(s) for comment submission. During the meeting, the Department will present the basis for adding or removing analytes ( i.e., including technical and scientific support for the proposed changes), as well as a discussion of related costs and benefits. This information will be provided in advance to DTAB members. The Department will review all submitted public comments and will share information during a DTAB session prior to DTAB’s review of SAMHSA’s recommendation to the Secretary regarding each proposed change.
The Department will make the final decision on any panel changes and include the effective date(s) in the annual Notice, to allow time for drug testing service providers ( e.g., immunoassay kit manufacturers, oral fluid collection device manufacturers) to develop or revise their products, and for HHS-certified laboratories to develop or revise assays, complete validation studies, and revise procedures.
Three commenters specifically agreed with the need to streamline and improve processes for making changes to the testing panels, but expressed concern over the process for testing panel review and who would be involved. These commenters suggested involving other stakeholders ( e.g., HHS-certified laboratories, DTAB, FDA). As noted above, the Department will use multiple methods and involve subject matter experts from various stakeholder groups to determine testing panel changes, and will provide opportunity for public review and comment before changes are made. FDA, DOT, and other Federal partners will have opportunities to review and provide input.
More Posts

Other Bodily Fluid House Hearing – HB-4391- Update 5-22-25
Michigan House HearingHB-4391 Saliva Test Update 5-22-25Watch the hearing or read the summary.Click here or image below to see videoFYI: Marijuana although voted to be legalized is still classified as a controlled substance in the State of Michigan and Federally. More...

Criminal Law FAQs – Marijuana Offenses
Michigan Criminal Laws FAQs Marijuana OffensesFAQ 1: Is recreational marijuana legal in Michigan? Answer: Yes, recreational marijuana is legal for adults 21 and over in Michigan. However, there are restrictions on possession, use in public places, and driving under...

Criminal Law FAQs – Operating While Intoxicated (DUI – OWI)
Michigan Criminal Laws FAQs Drunk Driving (Operating While Intoxicated - OWI)FAQ 1: What is the legal blood alcohol content (BAC) limit in Michigan? Answer: In Michigan, the legal BAC limit for operating a vehicle is 0.08% for individuals 21 years of age or older. For...

The Case of Cannarbor -v- The Michigan Dept of Treasury
Nice Try...This case centered on the disagreement between Cannarbor, Inc., a medical marijuana provisioning center operating in Michigan, and the Michigan Department of Treasury concerning the obligation to collect sales tax on the retail sale of medical marijuana....

Legal Tip – Driving High on Cannabis in Michigan
Driving under the influence of cannabis is illegal and carries serious consequences in Michigan.We have fought and won many cases from the District Courts, Circuit Courts, Court of Appeals and the Supreme Court through out the State of Michigan. We have also fought...
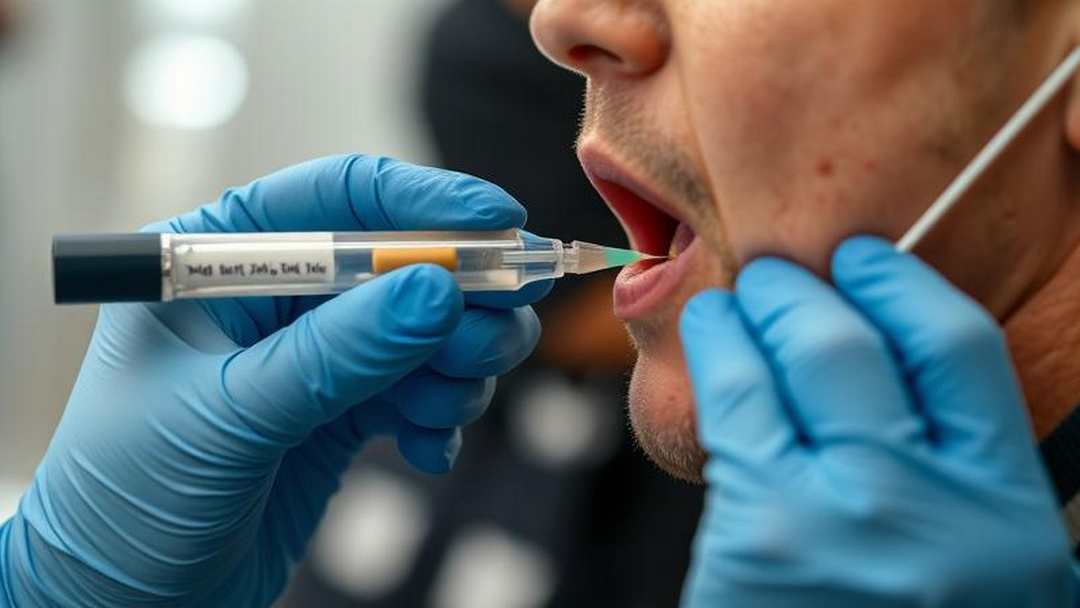
Michigan House Bill NO. 4391
It may just be easier to collect and analyze tears.This legislation seeks to integrate saliva testing for cannabis within law enforcement procedures, designating a refusal to participate in this testing as a criminal offense, similar to the penalties imposed for...

Legal Tip – Your Rights During a DUI Stop in Michigan
Komorn Law - Quick Legal TipsLegal Tip: Understanding Your Rights During a DUI Stop in Michigan A DUI stop can be stressful, but knowing your rights is crucial. You have the right to remain silent. You are not obligated to answer questions beyond basic identification....

How to create and share a Dropbox link
Simplified Sender and Receiver Dropbox Share Instructions to Someone NOT on your Team. Don't get caught up in another license or give access to your whole box by mistake.Dropbox Sender Share Instructions Log into your Dropbox account Hover over the file or folder...

Smell of marijuana no longer legal grounds for search
The Michigan Supreme Court has ruled that the smell of marijuana alone is no longer sufficient probable cause for police to conduct a warrantless search of a vehicle. This decision overturns a previous precedent where the odor of marijuana was considered enough...

Michigan Forfeiture News Articles
Can the police sieze your belongings and hold it without charging you with a crime?Civil asset forfeiture is a legal process that allows law enforcement agencies in Michigan to seize property they suspect is connected to criminal activity, even if the owner hasn't...










